- BACTEROMIC
Automatyczny system do określania lekowrażliwości (AST) oparty o technologie mikroprzepływowe
- PROBLEM
Antybiotykooporność bakterii
Antybiotykooporność to wszechobecny problem i poważne zagrożenie dla zdrowia publicznego – każde zastosowanie antybiotyku może doprowadzić do rozwoju oporności na antybiotyki. Bakterie coraz szybciej stają się oporne na działania nowych antybiotyków.
- FAKTY
Co 15 minut na świecie ktoś umiera z powodu infekcji wywołanej bakterią oporną na antybiotyki
- Każdego roku antybiotyki chronią przed śmiercią miliony osób i pozostają głównym sposobem leczenia zagrażających życiu infekcji bakteryjnych.
- Duża liczba niepoprawnie przepisanych, nadużywanych antybiotyków doprowadziła do antybiotykooporności, która stała się globalnym problemem dla ochrony zdrowia. Każdego roku z powodu antybiotykooporności umiera 700 000 osób na świecie.
- Przypuszcza się, że do 2050 roku z powodu antybiotykooporności może umrzeć 10 milionów ludzi. Antybiotykooporność może stać się częstszą przyczyną śmierci niż nowotwór.
Antybiotykooporność to wszechobecny problem
50% antybiotyków używanych w szpitalach jest zwykle zlecanych niepotrzebnie lub niewłaściwie. Choć antybiotyki ratują życie, każde ich zastosowanie może prowadzić do rozwoju antybiotykoopornych bakterii u ludzi. Walka z antybiotykoopornością toczy się wszędzie.
Bakterie są wszechobecne i przenoszą się między organizmami
W naturalnych skupiskach bakterii, jakimi są np. placówki medyczne, bakterie przenoszą się między pacjentami i personelem, często utrudniając leczenie.
Bakterie przenoszą się między ludźmi i zwierzętami w każdym miejscu i przy okazji każdej codziennej aktywności.
Infekcje bakteryjne są możliwe w każdym miejscu publicznym oraz środkach transportu, lokalnych, jak i międzynarodowych.
Bakterie znajdują się w każdym pożywieniu.
Walka z bakteriami wymaga dokładnego rozpoznania

- NASZE ROZWIĄZANIE
Najlepszą odpowiedzią jest precyzyjna diagnostyka
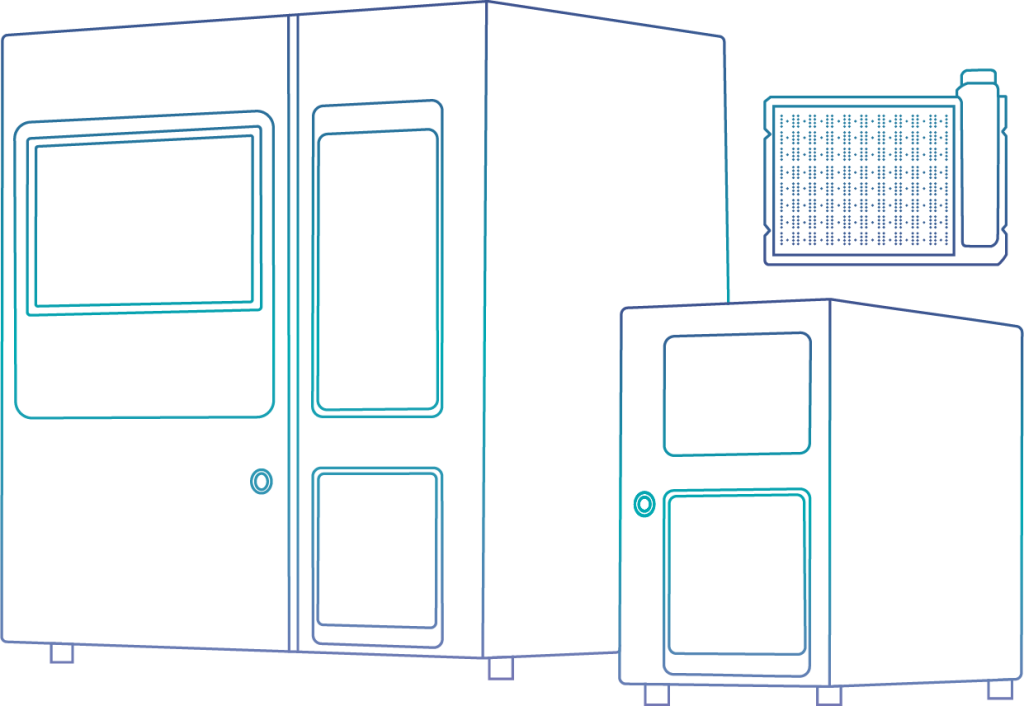
- NASZE ROZWIĄZANIA
Najlepszą odpowiedzią jest precyzyjna diagnostyka
Badanie wrażliwości na środki przeciwdrobnoustrojowe (antybiogram) jest skutecznym rozwiązaniem w walce z antybiotykoopornością
BACTEROMIC jest systemem diagnostycznym odpowiadającym na jedno z najważniejszych wyzwań współczesnej medycyny – wielolekooporność. System umożliwia fenotypową ocenę skuteczności działania antybiotyków, wykorzystywanych w leczeniu m.in. zakażeń układu oddechowego, krwi, dróg moczowych czy skóry. Posiada także zdolność do wykrywania mechanizmu oporności.
Dzięki determinacji i wiedzy zespołu biotechnologów, inżynierów i programistów oraz innowacyjnemu podejściu do problemu współczesnej diagnostyki, powstało urządzenie o bardzo dużym potencjale rynkowym, które ma szansę zrewolucjonizować leczenie antybiotykami.
Nasza misja
Tworzymy i dostarczamy najbardziej zaawansowany system testowania wrażliwości na środki przeciwdrobnoustrojowe, który rewolucjonizuje leczenie infekcji bakteryjnych dzięki szybkim i kompleksowym informacjom. Jednocześnie aktywnie zwiększamy globalną świadomość i zaangażowanie w zwalczaniu oporności na antybiotyki w celu szybkiego wyleczenia infekcji bakteryjnych u każdego pacjenta na świecie.
Nasza wizja
Świat, w którym każdy pacjent otrzymuje optymalne i niedrogie leczenie dzięki szybszym i dokładniejszym testom wrażliwości na środki przeciwdrobnoustrojowe.



Projekt uzyskał dofinansowanie z programu badań i innowacji HORIZON 2020 Unii Europejskiej w ramach umowy nr 881101.

Uzyskanie międzynarodowej ochrony patentowej wynalazków „Chip mikroprzepływowy oraz Segment inkubacyjny”
Aktualności
- Sprawdź najnowsze informacje
Formularz kontaktowy


